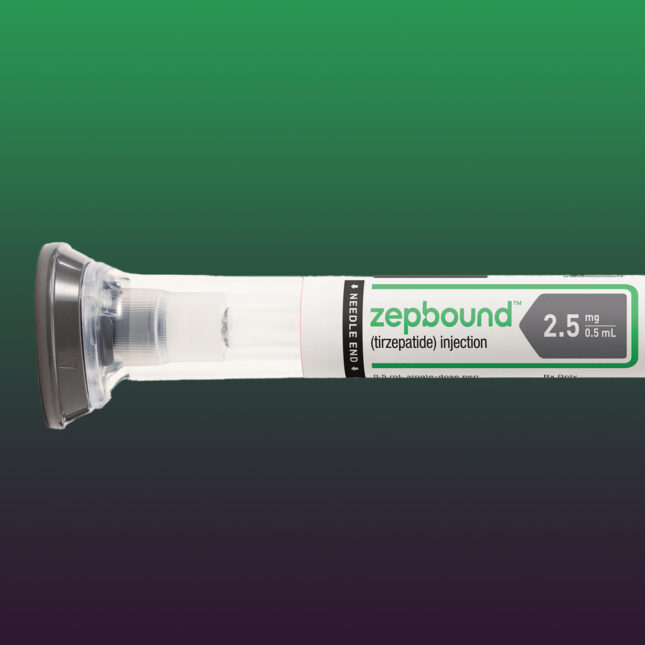
The Food and Drug Administration on Thursday confirmed that a shortage of Eli Lilly’s obesity drug tirzepatide has been resolved, a move that will soon put a stop to companies making cheaper copies of the injection.
The agency said it would give these compounders a grace period of 60 to 90 days before enforcing rules that would put a halt to their work, in an effort to avoid disruption for patients.
In early October, the FDA pulled Eli Lilly’s tirzepatide — sold as Mounjaro for diabetes and Zepbound for obesity — off its drug shortage list after nearly two years. That should have prohibited compounding pharmacies from continuing to make copies of the drug, as they’re allowed to do so only when a treatment is on the shortage list. But after the trade group the Outsourcing Facilities Association took the FDA to court, the agency made a sudden about-face, saying it would reconsider its decision and allow compounders to continue for the meantime.
This article is exclusive to STAT+ subscribers
Unlock this article — plus daily coverage and analysis of the pharma industry — by subscribing to STAT+.
Already have an account? Log in









